Cnn
–
The food administration and the drug of the US modified The terms of use of emergency and modern and modern’s use of the PFIZIA and modern age 65 and certain people with doses debilitate before this addicted vaccination.
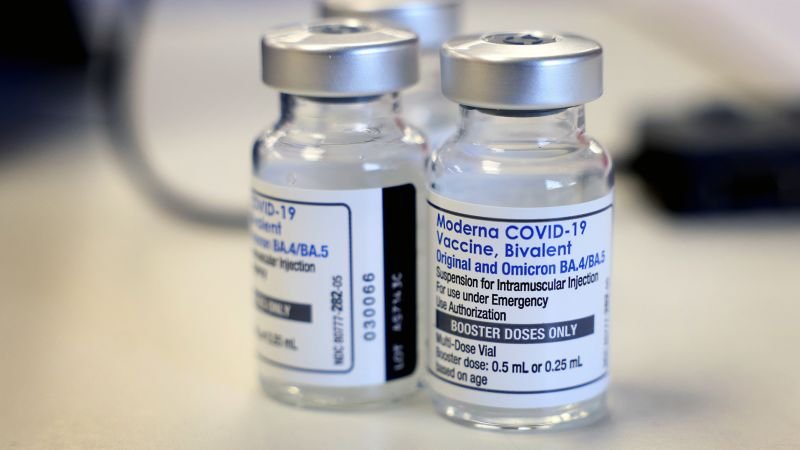
The bivalent cattle are made from pfizer and modern wear instructions for the original fight of the viruses of 19 € and the omicer and their spinoffs.
Were available in the United States from September in an emergency use authorizations, or the eu, that restricts well as cattle.
Tuesday, FDA has changed the terms of the authorizations for those cattle so that certain individuals could take a further dose in front of most.
Name, adults from 65 and older that they received a single dose of a bivalant vaccine may receive an additional dose at least four months later.
Most individuals with certain immunocompromise grades that received a first dose of a bivalent can get a second at least 2 months later. Additional doses can be administered to the discretion of their health provider.
The dr. Peter Hoez, who’s in the center for the development of the Texas Children’s Hospital, called the FDA to increase Bivalent Boosters for those who want them. It says for most, today’s guide from agency makes sense.
“My only question is why the age of 65 years? What was this based? Ordinally would you rather have to give you 60 or even 50,” Humez finger in an email to CNN.
“For those Americans who understand their importance, we should give you a dead boosters. We will be able to drop on another annual gaze. The information comes up some time the summer.”
For the immunishnized kids at 6 months to 4-year-olds, the eligibility for additional bivalctents dose depends on previously received vaccine, the FDA said in a release.
Another biggest change is that the most inappropriate individuals can now receive a single dose of a bivalent vaccine, rather than original cattle, the said agency. The fairy-summoned the recommendation for unfair individuals later
«Evidence now is available that the most of age and more in-cov-cov-4, nail from the rate of the covida-19, be the infection that can serve a Foundation for protection by protecting the bivalent cattle. Covid-19 continues to be a very real risk for a lot of people, and we encourage you in advance to consider to be running with the vaccination with a kiss of the Batty 19. The most serious data is going to prove the youngest findings, which are explain, which are explained, having the Hospitalization and death, ‘Second of Bicycles and research, in a Release of the news. I am
The children 6 months for 5 years that they have not yet to have two dose of the bivalent modern dose, or a series of three bivaletative donos if they are 6 months through 4 years of years old. The children who are the age of the age of the two doses of the Bivalent modern-bioser-bioncer-bioncer-bioncer-bioncer-bioncer’s vaccine.
The children 6 months for 5 years that have been commenced in their dose, you can now a dose a dose vaccination, but the dose number must depend on that dose number.
The agency stressed that most people who have got a dose of a bivalent cavy are not currently eligible for a second dose.
And they are all the way they have not yet farther than the first dose of a bivalent vital to do, and several Americans are always on that bucket.
Only about 17% of the eligible, fewer than 1 in 5 Americans had achieved a recommended dose.
Since time has passed, adults with the immune feature reduced because of their age or an underlying health issue were asked to the other dose of the bival cattle.
The US Center for the center of the prevention and prevention has claimed the anticipated data that the effectives won the cynications mervals, has already started at Wane.
But the agency is not free to do what is known as a “Subjects allowed to the Boosters, allowing doctors to offer additional doses because of the euau eua.
The updated terms give the CDC of design on Immunization practices (ACIP) larger to recommend additional doses of bivalent cattle. ACIP is holding a meeting on the 19cans of the 19 cascades and is expected to approve of the FDA.
For everyone has covered by changes, the fDA says they are coming to make decisions of vaccinee vaccinee after the exposition of the fall in June.
Tri Canada and the United Kingdom They offered another round of bivalent boosters to those in more risk from covit-19 this spring.